Anti-PD-L1 from Rabbit (HL1041) – unconj.
-
Overview
SKU IHC-184010 Specificity Species Reactivity Immunogen Carrier-protein conjugated synthetic peptide encompassing a sequence within the C-terminus region of human PD-L1, The exact sequence is proprietary.
Host Species Isotype Clone Clonality (Mono-/Polyclonal) Application Immunohistochemistry (frozen sections), Immunohistochemistry (IHC), Immunohistochemistry (Paraffin-embedded Sections)
Conjugation Format Product line / Topic Intended Use Temperature - Storage Temperature - Transport Manufacturer / Brand - Datasheets and Downloads
-
Additional Product Information
PD-L1 antibody clone HL1041 validated for PD-L1 immunohistochemistry (IHC FFPE) on tumor tissue
Recombinant rabbit clone HL1041 has been validated for the specific detection of PD-L1 in paraffin-embedded human tissue specimen by routine immunohistochemistry and does not cross react with PD-L2.
Alongside the importance in routine pathology, PD-L1 is being investigated as a possible predictive marker for immune therapy and plays a central role for basic research of the tumor microenvironment and the cancer immunology checkpoint.
PD-L1 is expressed in various human tumors with highest frequencies in head and neck, cervical, glioblastoma multiforme (GBM), bladder, esophageal, (triple-negative) breast cancer, hepatocarcinoma and CUP (Cancer of Unknown Primary) cancer. PD-L1 expression is associated with tumor grade, squamous histology, immune cell density.
Physiologically, PD-L1 acts as the major ligand for PD-1 (Programmed cell death-1). PD-1 is a co-inhibitory receptor expressed on lymphoid and non-lymphoid-derived cells that negatively regulates peripheral T-cell responses. Activated T cells have the ability to recognize and bind cancer cells. The bound effector T cells release cytotoxins, which induce apoptosis in cancer cells.
PD-L1 expressed on tumor cells binds to PD-1 receptors on the activated T cells, which leads to the inhibition of the cytotoxic T cells and deactivated T cells remain inhibited in the tumor microenvironment. Thus, the PD-1/PD-L1 pathway represents an adaptive immune resistance mechanism exerted by tumor cells to escape immune response.
New therapies blocking PD1/PD-L1 interaction have revolutionized cancer immune therapy. Monoclonal antibody therapies against PD-1 and PD-L1 are being routinely used including:
Nivolumab, an anti-PD-1 drug developed by Bristol-Myers Squibb, approved for previously treated metastatic melanoma and squamous non-small cell lung cancer.
Pembrolizumab, developed by Merck is approved for previously treated metastatic melanoma.
PD-L1 is being investigated as a possible predictive marker for anti-PD-1 therapy.
PD-L1 antibody clone HL1041 validated for PD-L1 immunohistochemistry (IHC FFPE) on tumor tissue
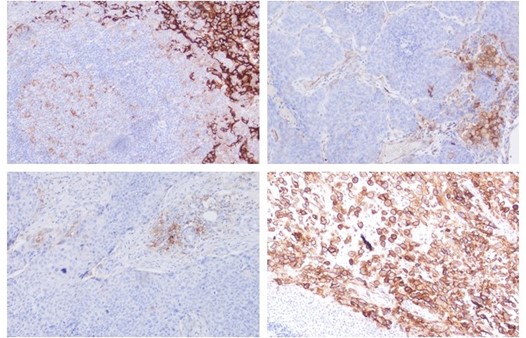
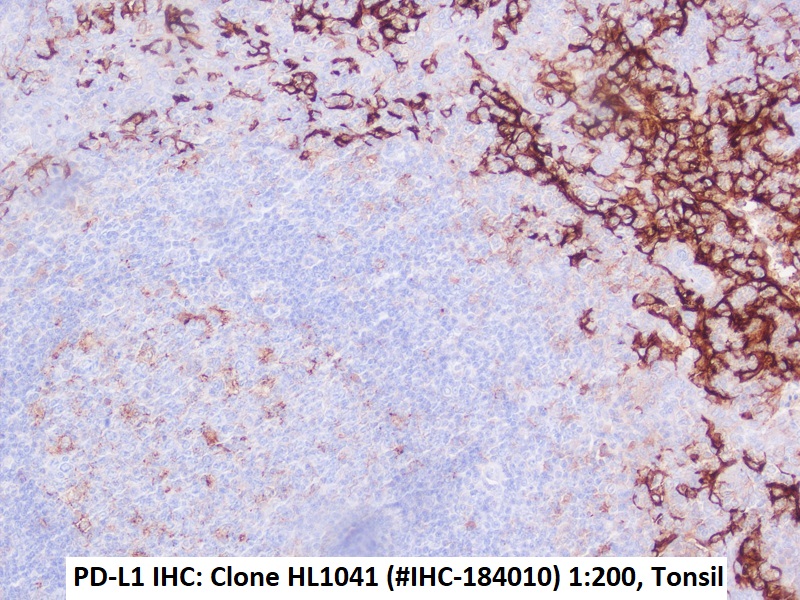
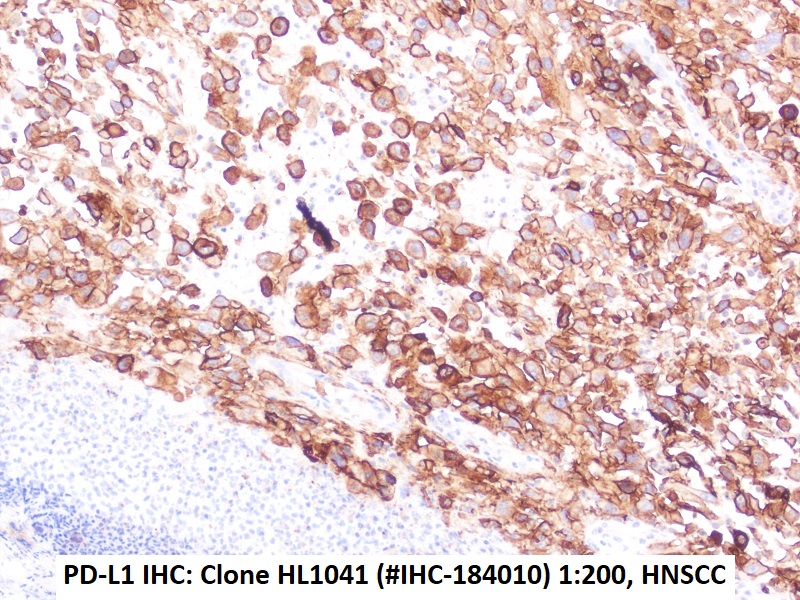
Figures: Immunohistochemistry of PD-L1 clone HL1041 in formalin-fixed paraffin-embedded human tonsil and HNSCC sections: Tonsil (upper Left) and HNSCC (Upper right, Lower left, Lower right); 10′ HIER pH 6.0, 1:200. (Pictures courtesy of D. Dietrich, Department of Otorhinolaryngology, University Medical Center Bonn (UKB), Germany) -
Images

Immunohistochemical detection of PD-L1 with anti-PD-L1 Antibody clone HL1041 in formalin-fixed paraffin-embedded human tonsil 
Immunohistochemical detection of PD-L1 with anti-PD-L1 Antibody clone HL1041 in formalin-fixed paraffin-embedded HNSCC Section
