ONCOdianova![]() developes new antibodies for IHC detection of cancer immunology checkpoint biomarkers in FFPE-tissues. ONCOdianova’s vision is to provide the best antibodies for IHC analysis of biomarker expression profiles to better understand the complex interaction of immune cells and tumor cells in patient tissue. ONCOdianova believes that multiplexed IHC-assessment of immune checkpoint targets in tumor tissues is indispensable for cancer research.
developes new antibodies for IHC detection of cancer immunology checkpoint biomarkers in FFPE-tissues. ONCOdianova’s vision is to provide the best antibodies for IHC analysis of biomarker expression profiles to better understand the complex interaction of immune cells and tumor cells in patient tissue. ONCOdianova believes that multiplexed IHC-assessment of immune checkpoint targets in tumor tissues is indispensable for cancer research.
A growing number of i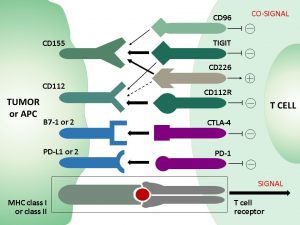 mmune checkpoints emerge as targets for anticancer therapy. Cancer cells and the cells of the surrounding microenvironment have been shown to upregulate the expression of components to suppress the antitumor immune response by generating coinhibitory signals. So far, PD-1 and CTLA-4 emerged as the most successful immune targets for anticancer therapies. The recently discovered TIGIT pathway provides significant therapeutic promise, particularly in combination with other immune checkpoint inhibitors. Dianova’s antibody development program concentrates on monoclonal antibodies against immune checkpoint targets for immunohistochemical application in standard FFPE human tumor tissues for diagnostic purposes.
mmune checkpoints emerge as targets for anticancer therapy. Cancer cells and the cells of the surrounding microenvironment have been shown to upregulate the expression of components to suppress the antitumor immune response by generating coinhibitory signals. So far, PD-1 and CTLA-4 emerged as the most successful immune targets for anticancer therapies. The recently discovered TIGIT pathway provides significant therapeutic promise, particularly in combination with other immune checkpoint inhibitors. Dianova’s antibody development program concentrates on monoclonal antibodies against immune checkpoint targets for immunohistochemical application in standard FFPE human tumor tissues for diagnostic purposes.
Legend: T cell receptors are targets for cancer immunology checkpoint blockade.
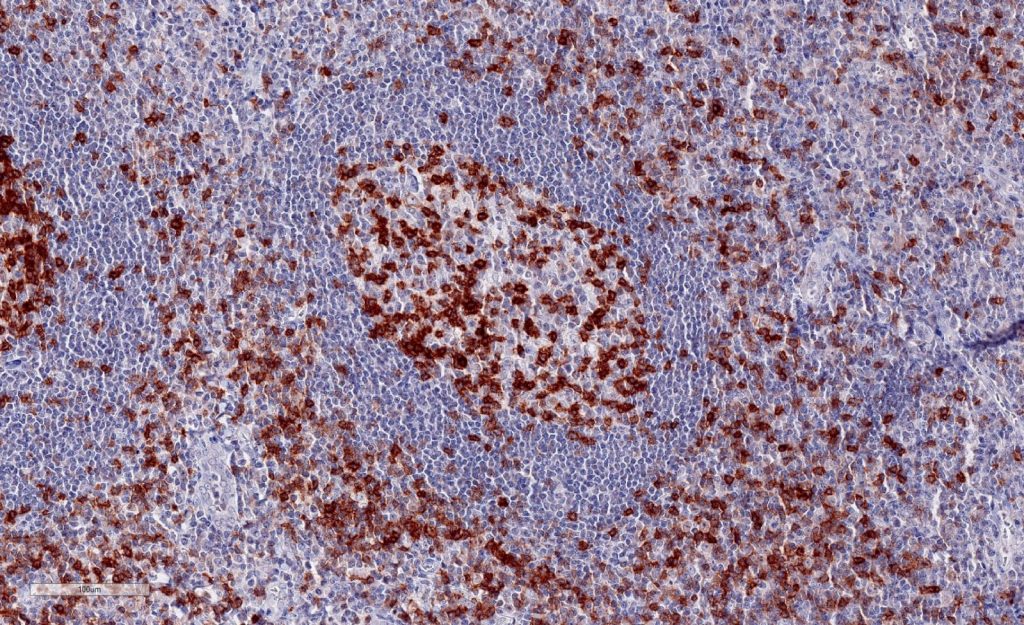
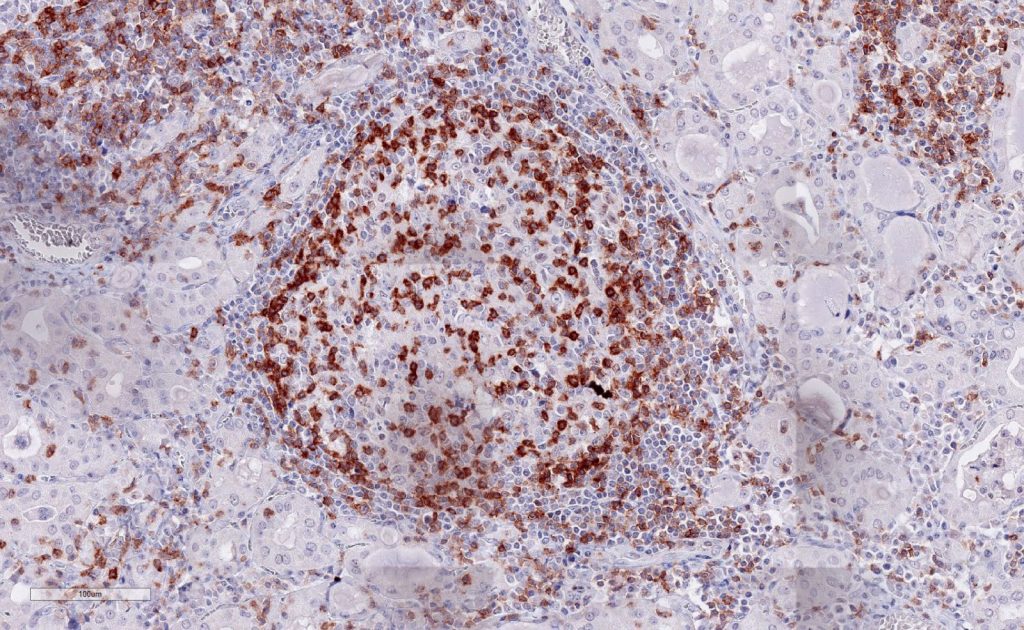
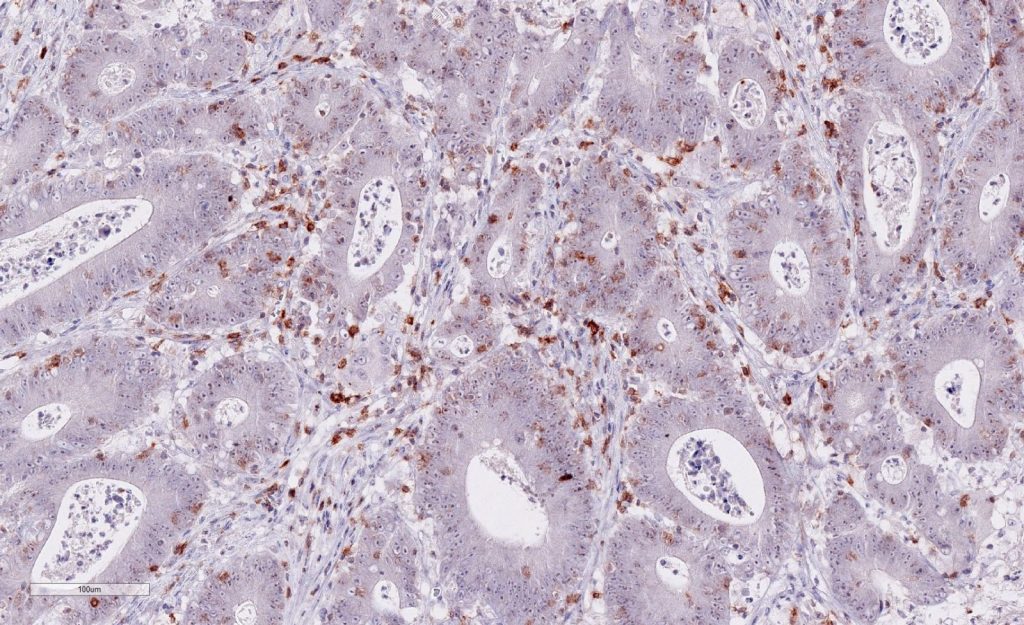
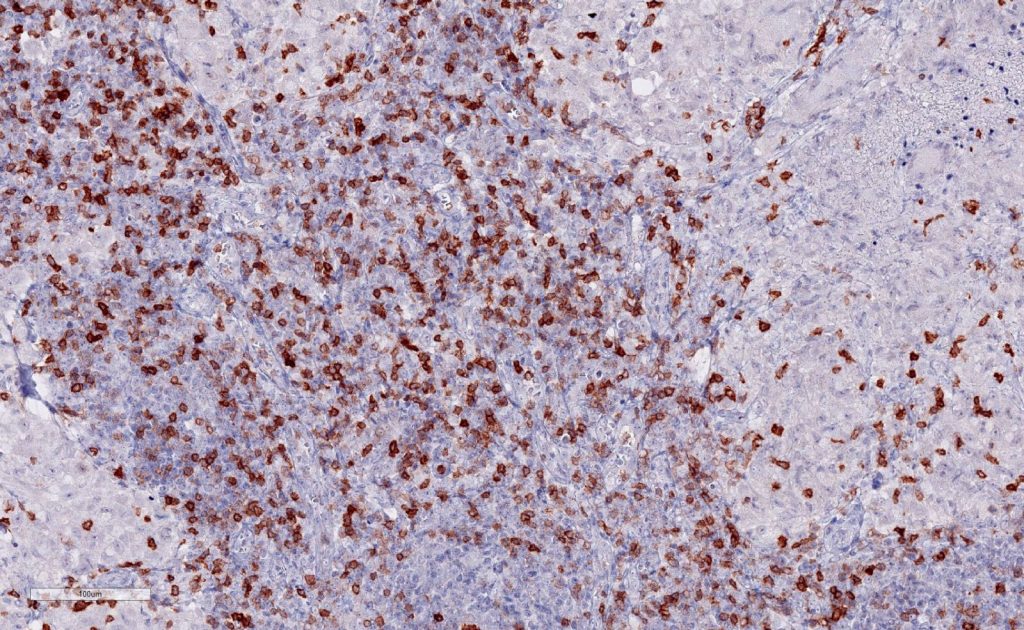
Immunohistochemistry of human TIGIT in formalin-fixed paraffin-embedded tissue sections
Clone TG1 is the first monoclonal antibody detecting TIGIT (T cell immunoreceptor with Ig and ITIM domains) in standard FFPE human tissue specimen. It has been validated for the identification of TIGIT positive T-cells infiltrating human tumors in order to allow the detection of TIGIT in the tumor microenvironment under pathological conditions. Immunohistochemical application of monoclonal antibody TG1 may provide valuable information for clinical research and potential therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint.
(pictures courtesy of Andrea Hinsch and Niclas Blessin, Institute of Pathology, University Hospital Eppendorf (UKE), Hamburg, Germany).
Strong membrane staining of TIGIT-positive lymphocytes in human FFPE tissues with monoclonal antibody TG1.
Each image is shown at 20x magnification. Sections were stained for TIGIT (T cell immunoreceptor with Ig and ITIM domains) antibody using a HRP conjugated polymer system with DAB as a chromogen (brown) and counterstained with haematoxylin (blue).
TIGIT – Scientific background
The immunoreceptor TIGIT acts as an inhibitory immune checkpoint on both T cells and natural killer (NK) cells by a highly complexe pathway. Known ligands for TIGIT include CD155, and CD112. The TIGIT/CD155/CD112 network also interacts with other checkpoint regulators (see scheme above).
TIGIT expression on CD8+ T cells and other lymphocytes is highly correlated with the expression of other coinhibitory molecules, including PD-1. The complexities of the TIGIT pathway upregulated in inflammation and in cancer tumor and its interactions with other inhibitory checkpoint pathways offers opportunities for research and clinical translation. We believe that monoclonal antibodies for reliable and standardized histopathological detection of immune checkpoint targets in tumor tissues will be crucial and indispensable for future studies and diagnostic approaches.
References for clone TG1
- Blessin NC et al. Patterns of TIGIT expression in normal lymphatic tissue, inflammation and cancer. Disease Markers, Volume 2019, Article ID 5160565, 13 pages, https://doi.org/10.1155/2019/5160565
- Li W et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018, 18:1209, https://doi.org/10.1186/s12885-018-5111-1
- Blessin NC et al. Abstract 696: Patterns of TIGIT expression in normal lymphatic tissue, inflammation and cancer. Pro-ceedings: AACR Annual Meeting 2018; April 14-18, 2018; Chicago, IL., DOI: 10.1158/1538-7445.AM2018-696, July 2018
- Simon R et al. Abstract 2142: High variability of TIGIT expression in Hodgkin’s lymphoma. Proceedings: AACR Annual Meeting 2018; April 14-18, 2018; Chicago, IL, DOI: 10.1158/1538-7445.AM2018-2142 Published July 2018
General references:
- Blake SJ et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188. doi:10.1158/1078-0432.CCR-16-0933 .
- Grogan J et al. The immunoreceptor TIGIT regulates anti-tumor immunity. J Immunother Cancer. 2016:4(suppl 1):P209.
- Kurtulus S et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053- 4062. doi:10.1172/JCI81187 .
- Lozano E et al. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869-3875. doi:10.4049/jimmunol.1103627 .
- Pauken KE, Wherry EJ. TIGIT and CD226: tipping the balance between co-stimulatory and coinhibitory molecules to augment the cancer immunotherapy toolkit. Cancer Cell. 2014;26(6):785-787. doi:10.1016/j.ccell.2014.11.016
- Stanietsky N et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858-17863. doi:10.1073/pnas.0903474106 .