dianova.com is the portal for secondary antibodies and conjugates from BIOZOL. In our secondary antibodies search you will find more than 7000 secondary antibodies. This online guide will help you to find the right secondary antibody for your application.
In our antibody search, you can select the properties you need by choosing from 6 properties in drop-down menus, allowing you to quickly and easily find the products best suited to your needs.
You have additional questions? Our scientific consulting team will be happy to help you.
We are happy to advise you at any time.

Content
For the optimal selection of secondary antibodies, some basic information from the field of immunology is helpful. You will find this information in the first two sections of this tutorial.
Antibody Structure
A brief introduction – what you should know about the structure of antibodies.
What are secondary antibodies ?
What is a secondary antibody? What are the advantages of using secondary antibodies over other detection methods?
Step 1: Target Species
Primary antibodies from over 20 species can be detected with our selection of secondary antibodies.
Step 2: Host Species
In addition to secondary antibodies from goat and donkey, 9 other species are available for selection.
Step 3: Antibody Format
The format defines which molecular shape the secondary antibody has. Fab fragments are only suitable for specific applications.
Step 4: Antibody Specificity
The specificity defines which part of the primary antibody is recognized by the secondary antibody.
Step 5: Label
The choice of the reporter molecule linked to the secondary antibody allows its use in different applications.
Step 6: Minimal Cross-Reactivity (MinX)
Adsorption prevents cross-reactivity with primary antibodies of other species and is a prerequisite for successful multiple labeling
Step 1: Target Species
Against which species should the secondary antibody be directed?
This is usually the species from which the primary antibody originates. Only in a few exceptional cases is it useful to use cross-reacting antibodies against a related species.
Antibodies against hamster IgG
Nearly all hamster monoclonal antibodies are derived from mouse Armenian hamster hybridomas. Thus, the IgG produced by these hybridomas are “Armenian” and not “Syrian” IgG. Most commercially available polyclonal anti-hamster IgG antibodies are directed against Syrian hamster IgG and therefore do not detect Armenian hamster monoclonal antibodies as effectively as anti-Armenian hamster IgG.
Detection of antibodies from sheep, goat and bovine
There is extensive homology between IgG’s from sheep, goat and bovine, so that secondary antibodies generated against goat can also be used for the detection of primary antibodies from sheep/bovine. Anti-sheep antibodies are also suitable for the detection of primary antibodies from goat/bovine.
Step 2: Host Species
From which host species should the secondary antibody be derived?
Experience has shown that antibodies from goat or donkey are suitable for the detection of primary antibodies of most species.
In the case of indirect multiple labeling with unconjugated primary antibodies, all secondary antibody conjugates should be from the same host species to avoid cross-reactions between secondary antibodies. Strongly preadsorbed antibodies (see also step Adsorption) from the donkey are more specific than the less adsorbed secondary antibodies from other host species, but for this reason they may also be less sensitive.
In case of multiple labeling, all secondary antibodies should be from the same species!
In the case of multiple labeling, the
secondary antibody should be adsorbed against all primary antibody species that are also detected (see step Adsorption)!
To reduce background, normal serum from the same species as the secondary antibodies originates from is best suited for blocking!
Bovine Antibodies for the detection of goat Ig
Reagents used in the laboratory often contain bovine immunoglobulins. This applies, for example, to FCS in cell culture media, milk powder in Western blot applications or BSA as a stabilizer for proteins and antibodies. When detecting primary antibodies from goat, this can cause problems due to the close relationship of goat and bovine. For detection of goat primary antibodies without cross-reaction to bovine, we recommend bovine anti-goat antibodies from Jackson ImmunoResearch (805-xxx-180).
Antibodies from alpaca
Antibodies from alpaca are also suitable as secondary antibodies if reagents contain bovine IgGs. In addition to conventional IgG1 (classical structure), IgG2 and IgG3 are present in the serum of camelids. These have neither light chains nor CH1 domains in the heavy chains (see section Secondary antibody structure). These heavy chain antibodies have a small size, are extremely stable and show high specificity and affinity for the respective antigen. These properties make them particularly suitable for use as secondary antibodies.
Immunoprecipitation with rabbit antibodies
If antigen-antibody complexes are to be separated from other components by means of protein A agarose, preferably rabbit antibodies are used. These bind better to protein A than antibodies from goat or other species. IgG from goat, sheep and donkey (but also from rabbit) bind well to protein G.
Step 3: Antibody Format


Proteolytic pretreatment of antibodies with papain results in cleavage into two monovalent Fab fragments and one Fc fragment. Proteolytic treatment with pepsin results in a truncated Fc fragment and a bivalent F(ab’)2 fragment.
Structure of the secondary antibody: whole molecule (H+L), F(ab’)2- or Fab fragment?
IgG whole molecule
The total IgG molecule is the most commonly used antibody form and is the most suitable for the majority of applications.
Enzymatic digestion of total IgG molecules (see figure) yields antibody fragments that have special properties and that are in some applications more suitable as detection reagents than total molecules.
F(ab’)2 fragments
F(ab’)2 fragments are used when it is desired to prevent the secondary antibody from binding to Fc receptors on cell surfaces to avoid background staining. They have better diffusion properties because they are smaller than whole molecules and cannot aggregate via their Fc part. Both properties may be advantageous in certain applications.
The use of F(ab’)2 fragments does not prevent binding of the primary antibody to Fc receptors! Blocking with normal serum derived from the same host species as the secondary antibody is not always successful in this case. In the case of background caused by Fc receptors, you can block with IgG or IgG fragments of the species from which the sample material originates and work with a secondary antibody preadsorbed against this species.
Monovalent Fab-Fragments
Monovalent Fab fragments are used to block and double label primary antibodies from the same species or as blocking reagents against endogenous immunoglobulins in cell and tissue stains where tissue and primary antibodies are from the same species. Since Fab fragments have only one antigen-binding site (monovalent), once they have bound their target molecule, they cannot bind any other antibodies
Antisera
Antisera against whole IgG molecules (anti-IgG (H+L)) are recommended mainly for use as bridging antibodies in the PAP and APAAP methods.
Step 4: Antibody Specificity
What should the secondary antibody detect?
For immunological assays, mainly polyclonal antibodies of the IgG type (e.g. from rabbit, goat, chicken, etc.) or monoclonal antibodies from mouse, rat or hamster, which are obtained from B-cell hybridomas, are used. Secondary antibodies should have a high sensitivity for this primary antibody.
As a rule, this is a secondary antibody for which a whole molecule IgG(H+L) was used as the immunogen. This reagent recognizes a variety of epitopes on antibodies of different classes and subclasses. (see figure antibody structure)
If only one primary antibody is to be detected in a planned assay and immunoglobulins of other classes or subclasses are not a confounding factor, as is the case in most applications, specificity IgG (H+L) is usually most appropriate.
Frequently, several primary antibodies from different species are to be detected simultaneously in one experiment (multiple labeling). In this case, cross-recognition must be prevented. This is done by adsorption of antibodies. In this case the optimal specificity for each of the secondary antibodies is still IgG(H+L).
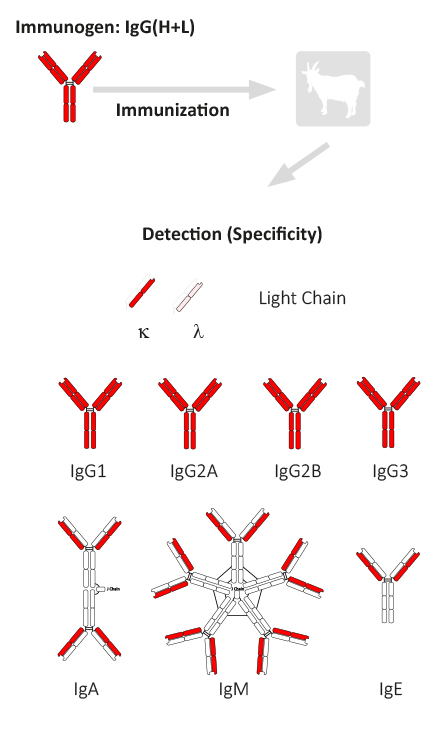

Generation of an antibody with a specificity mouse IgG (H+L)
The animal host species (e.g. goat) in which the antiserum is made is immunized with purified immunoglobulin or immunoglobulin parts of the corresponding target species. As a rule, the immunoglobulins used for immunization are obtained from total serum, i.e. the composition of the individual subclasses and parts corresponds to the normal distribution in the serum of the species.
Antiserum from animals immunized with IgG(H+L) contains antibodies directed against both the light and heavy chains of the IgG molecule. This antiserum reacts with both the F(ab’)2 part and the Fc part of IgG. Anti-IgG therefore also reacts very well with other immunoglobulin classes (IgM, IgA, IgE), since they have the same light chains (κ and λ). Anti-IgG (H+L) antibodies that are not preadsorbed (see step 6) have about 40% – 60% reactivity with light chains. This can be much lower for highly adsorbed anti-IgG (H+L) antibodies. For example, catalog no. 705-XXX-147 binds only 9% – 30% to light chains.
What are special cases for specificity selection?
Here you will find some examples of special cases. However, these examples only cover a small range of the specificities and more common applications available from us. With more than 70 choices in specificity / detection, our online search offers you many more possibilities.
Class specific antibody detection
Detection of class-specific antibodies in the presence of interfering antibodies of other classes or simultaneous detection of different antibody classes.


anti-IgG-Fc-Fragment
Immunization (e.g. of goat) with purified IgG Fc fragment (e.g. from mouse/human) leads to antibodies that specifically detect the heavy chain of the IgG molecule. Since the differences between the various immunoglobulin classes lie in the heavy chain region, IgG, but not IgM, IgA and IgE, can be detected with these antibodies. The Fc fragment-specific antibodies are additionally preadsorbed against F(ab’)2 fragments. In some cases, a possible cross-reaction against IgM and IgA (anti-human) or only against IgM (anti-mouse, anti-rat) is also minimized by further preadsorption(s). These antibodies (anti-human, anti-mouse, anti-rat) are identified by “Fcγ”. They have less than 1% residual activity against light chains or IgM or IgA.


anti-IgM (µ-Chain), anti-IgM Fc5µ
To produce IgM-specific antibodies, the host species (e.g. goat) is immunized with purified IgM (mouse and rat) or trypsin-digested, purified IgM Fc5µ (human). After affinity chromatographic purification, the anti-mouse and anti-rat IgM (µ-chain)-specific antibodies are adsorbed to IgG. The anti-human IgM Fc5µ are adsorbed against IgG and IgA. The anti-IgM specific antibodies usually have less than 1% residual activity against IgG or light chains.




anti-IgA (a-Chain) / anti-IgE (e-Chain)
Antibodies react with the heavy chain of IgA or IgE and are adsorbed against other subclasses, so that no significant cross-reactions with other classes such as IgG or IgM occur.
Detection of antibody subclasses
Detection of monoclonal antibodies of specific subclasses.
Simultaneous detection of monoclonal antibodies of different subclasses from the same species.


Subclass-specific anti-mouse antibodies against IgG1, IgG2a, IgG2b, IgG2c and IgG3
Due to their high specificity, these antibodies should only be used when mouse subclass discrimination is required.
For detection of mouse IgG subclasses with approximately equal sensitivity, we recommend the goat anti-mouse IgG (subclasses 1, 2a, 2b, and 3) Fcγ fragment-specific antibody (catalog no. 115-XXX-164), which has more balanced reactivity toward these subclasses.
Equal detection of subclasses



Because the antiserum (e.g., from goat) is predominantly directed against κ-type light chains, the sensitivity of anti-F(ab’)2 fragment antibodies in detecting primary antibodies carrying λ-type light chains is limited.
anti-IgG F(ab‘)2-Fragment
These antibodies are obtained by immunization with purified F(ab’)2 fragment of total IgG. Since the heavy chain portion of the F(ab’)2 fragment is only very weakly immunogenic, antibodies obtained in this way react, depending on their degree of preadsorption (see step 6), to more than 60% with light chain. Anti-F(ab’)2 fragment antibodies are additionally preadsorbed against IgG Fc fragment and therefore react only with the Fab part of IgG.
With these immunoreagents, all lg classes and subclasses (IgM, IgG1-4, IgA, etc.) can be detected with equal sensitivity. These antibodies are therefore ideally suited if all Ig classes regardless of subclass need to be detected or if it is not certain which class the primary antibody belongs to.




anti-IgG + IgM (H+L) / anti-IgG + IgM + IgA (H+L)
To achieve equal reactivity, these products are prepared by mixing different antibodies against each class. In a mixture of anti-IgG + IgM (H+L), the reactivity with light chains varies from 30% to 45%. The overall anti-IgM reactivity (including against light chains) in the mixture is approximately between 55% – 70%.
Detection of antibody light chains


Anti-IgG light chain specific antibodies react with native primary antibodies used for the detection of proteins in Western blots.
When diluted appropriately, they do not react with the reduced and denatured heavy chain (50 kDa) of the IgG molecule in the blot, for example in samples derived from immunoprecipitation (IP) experiments, where large amounts of denatured heavy chain may mask the target protein.
Anti-Light Chain antibodies have a strong reactivity with native IgG light chains, but the sensitivity with reduced/denatured forms may be decreased. They are not suitable for sensitive / quantitative detection of IgG light chains in Western blot.
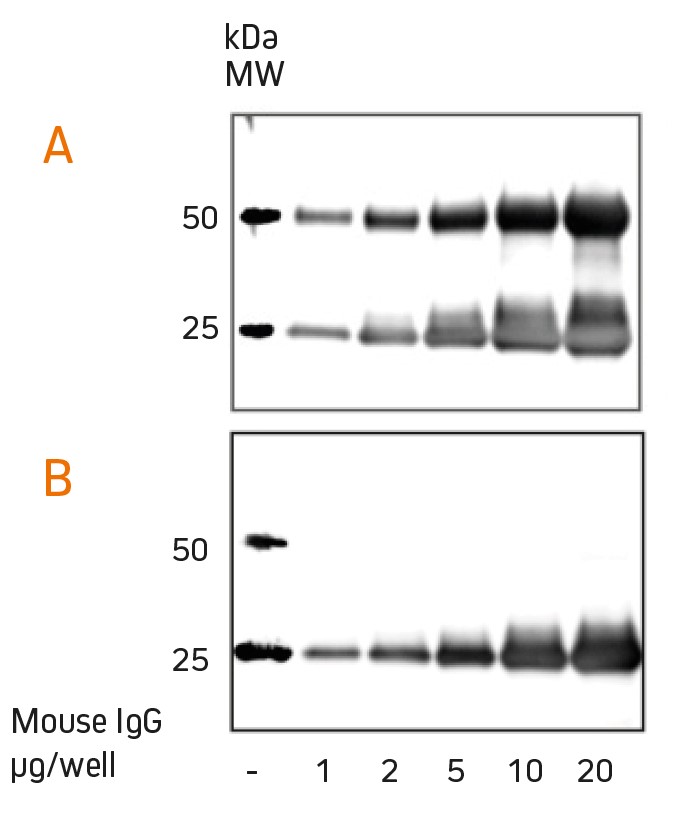

Detection of mouse IgG using anti-mouse IgG(H+L) (115-035-003) (A) and anti-mouse light chain specific antibody (115-035-174) (B). The light chain antibody allows detection of target proteins at 50 kDa that are otherwise masked (Fig. Jackson ImmunoResearch).
Step 5: Label
Which conjugation should the secondary antibody have?
The choice of conjugates depends on the method used and the desired type of detection (enzymatic, amplified, direct, etc.). For fluorescent dyes in particular, care must be taken to ensure that the necessary excitation sources and filters are available. For fluorescence-based methods in immunohistochemistry and cytochemistry, we generally recommend photostable dyes such as carbocyanines (e.g. Cy2, Cy3, Cy5) or AlexaFluor® in preference to traditional dyes (e.g. FITC, TRITC). Some of the dyes are also suitable for super-resolution microscopy. For STED the dyes Alexa Fluor 488, FITC and Alexa Fluor 594 are suitable, for STORM the dyes Alexa Fluor 488, FITC, Cy3, Alexa Fluor 647, Cy5 and Alexa Fluor 790 are suitable.
Secondary antibody dilutions
Reporter / Label for different applications and recommended dilutions
Fluorophores
Adjacent you will find links to resources about different fluorophores
Enzyme-conjugates
Adjacent you will find links to resources about different Enzyme-conjugates
Step 6: Minimal Cross-Reactivity (MinX)
What level of (pre)adsorption is required for my secondary antibody?
Ig-specific antibodies against a particular species can cross-react to a greater or lesser extent with immunoglobulins and serum proteins of other species. Therefore in some cases, antibodies are preadsorbed against immunoglobulins and serum proteins of one or more other species, largely eliminating cross-reactivity with molecules of that species (cross-reactivity < 1%). This is is called “minimized cross-reactivity” (MinX) or referred to as adsorbtion.
Adsorbed antibodies are recommended when the possible presence of immunoglobulins of another species in the assay may lead to cross-reactions.
If antibodies with different preadsorptions are available, it should be taken into account that antibodies adsorbed against closely related species sometimes show a strongly reduced epitope recognition and therefore recognize some monoclonal antibodies only weakly. This is especially true for anti-mouse IgG (adsorbed against rat), anti-rat IgG (adsorbed against mouse) or anti-(armenian)hamster IgG (adsorbed against mouse, rat).
Antibodies preadsorbed against closely related species may not react well with some IgG subclasses (especially IgG2b, IgG2c and IgG3). This is especially true for those subclasses that are largely homologous with the species against they were preadsorbed. For example, anti-mouse IgG preadsorbed against rat IgG should only be used if a primary mouse antibody is to be detected a) in rat tissue containing rat immunoglobulins or b) in other tissues if an additional primary antibody from rat is used.
Bovine serum albumin (BSA) and milk powder usually contain impurities of bovine immunoglobulins that react with anti-bovine, anti-goat, anti-horse, and anti-sheep IgG antibodies. Therefore, the use of BSA and/or dry milk to block or dilute these secondary antibodies may significantly increase background staining or reduce antibody titers. To circumvent this problem, the use of specifically IgG-free BSA from Jackson ImmunoResearch (e.g., Catalog No. 001-000-161) is recommended. Also to prevent cross-reactivity with milk powder, BSA or FCS, we recommend using bovine secondary antibodies (805-xxx-180) for the detection of goat primary antibodies.


6 Step Secondary Antibody Selection Guide (PDF Download)
This online guide is also available for download in our popular brochure “6 steps to the right secondary antibody”. The download sumarizes all information in a compact and printer friendly format.















