Specify and Intensify your Signals with Hapten-anti-Hapten Techniques
An important requirement for indirect multiple labelling of different biomolecules in one single tissue are primary reagents which act simultaneously and without cross reaction as target for secondary immunoconjugates. The underlying principle of this approach are variations in the structure of the primary detection reagents. Thus, primary antibodies from different host species or isotypes can be detected by species- or isotpype-specific secondary antibody conjugates.
Unfortunately, in some cases different primary antibodies are not available, or a lack of differences in structure, e.g. nucleic acid sequences, does not allow a specific detection by secondary antibodies. In such cases the so-called hapten-anti-hapten technique can be applied.
Haptens are very small molecules with a defined chemical structure. Without binding to carrier proteins haptens are not immunogen because of this size. However, they may be tagged as antigeneic determinates via anti-hapten-antibodies. Well known haptens are for example biotin, digoxigenin, and the fluorochrome fluorescein. By their chemical activated derivates (e.g. fluorescein isothiocynate [FITC], or active ester) haptens can be linked to nucleotides, antibodies, enzymes, or other biomolecules. The latter substances keep their specificity even following conjugation with haptens. Therefore, by using hapten-specific signals they can be detected with clear and distinct signals.
The primary detection reagent can not only be diagnosed specifically but quite often also with a much stronger signal. A further important advantage of hapten-anti-hapten detection methods is the low background noise and non-specific signal of relevant antigens as hapten like structures are not found on animal cells and tissues with the exception of biotin.
Antibodies for Hapten-anti-Hapten-Techniques
The most important applications for antibodies against haptens:
- Fluorescence in situ hybridization (FISH) for discriminative labelling of multiple probes
- In situ hybridization experiments where fluorescein- and digoxigenin-labelled oligonucleotides are applied simultaneously
- Detection and signal amplification of hapten-labelled primary antibodies
- Multiple labelling of hapten-conjugated primary antibodies (also possible with same host species)
With anti-fluorescein-, anti-digoxin-, and anti-biotin-conjugates from Jackson ImmunoResearch dianova offers a wide range of sensitive antibodies against different haptens for a large number of applications. Anti-hapten conjugates are usually applied as detection reagents in DNA-hybridization techniques, in immunohistochemistry, flow cytometry, ELISA-techniques or for the detection of hapten-labelled samples on blot membranes. Due to their excellent specificity they represent diagnostic tools in applications with a low signal to noise ratio or high background signals (e.g. by endogenous biotin). Also, they comfortably support analyses with multiple labelling approaches, analyses where a definite differentiation of relevant biomolecules is mandatory, and methods where the specific signal has to be amplified. For all of these applications a large variety of fluorescence-, enzyme-, and biotin-conjugated monoclonal mouse-antibodies against fluorescein, biotin, and digoxin are available.
> Anti-Hapten Conjugates in Immunohistological Staining
For histochemical detection in FISH probes and primary antibodies the so-called CARD- (catalyzed reporter deposition method) or the TSA-method (tyramide signal amplification technology) allows a powerful increase in sensitivity. In these two techniques peroxidise-labelled antibodies against haptens catalyze the accumulation of multiple tyramid-derivates of the target antigens where strong fluorescence signals are generated.
Anti-Hapten Conjugates in Fluorescence in situ Hybridisation (FISH)
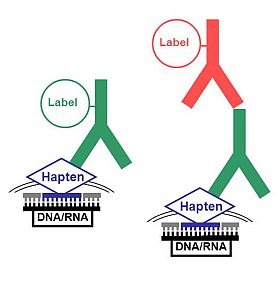
A most attractive approach for the use of anti-hapten-conjuagtes for the amplification of signals is the fluorescence in situ hybridization (FISH) technique. This technique is especially successful when multiple labelling is performed with different nucleic acid probes that are conjugated with fluorescein, biotin, or digoxigenin haptens. For a basic amplification hapten-labelled oligonucleic probes can be marked with conjugates from anti-hapten antibodies (Figure left).
Sometimes, however, a duplex amplification is necessary to intensify the signal. Here, non-conjugated anti-hapten antibodies can be applied which can then be detected by fluorescence-labelled secondary antibodies (Figure right). A further enhancement of the detection sensitivity is possible by using a fluorochrome-conjugated secondary antibody showing a specificity against the host species of the conjugate of the secondary antibody.
Product overview anti-Hapten-Conjugates for FISH
References – Application Examples
Anti-FITC and ABC/Biotin in double in situ Hybridization
Dirks RW, van Gijlswijk RP, Tullis RH, Smit AB, van Minnen J, van der Ploeg M, Raap AK, 1990.
Simultaneous detection of different mRNA sequences coding for neuropeptide hormones by double in situ hybridization using FITC- and biotin-labeled oligonucleotides.
J Histochem Cytochem. 38(4), 467-73.
Anti-FITC in ELISA
Harmer IJ, Samuel D, 1989.
The FITC-anti-FITC system is a sensitive alternative to biotin-streptavidin in ELISA.
J Immunol Methods 122, 115-121.
Anti-Digoxin in Western blotting
Härtig W, Kacza J, Paulke BR, Grosche J, Bauer U, Hoffmann A, Elsinghorst PW, Gütschow M, 2010.
In vivo labelling of hippocampal beta-amyloid in triple-transgenic mice with a fluorescent acetylcholinesterase inhibitor released from nanoparticles.
Eur J Neurosci. 31(1), 99-109.
Anti-Digoxin in Western blot analyses and IHC with haptenylated antibodies
Härtig W, Kirazov L, Brückner G, Holzer M, Gärtner U, Bigl V, 1997.
Blot analyses and immunocytochemistry of neural antigens with digoxigenylated primary and secondary antibodies.
Brain Res Brain Res Protoc. 2(1), 35-43.
Anti-Digoxin in IHC double staining of haptenylated primary antibodies
Härtig W, Brückner G, Holzer M, Brauer K, Bigl V, 1995.
Digoxigenylated primary antibodies for sensitive dual-peroxidase labelling of neuronal markers.
Histochem Cell Biol. 104(6), 467–472.
Anti-FITC and Avidin/Biotin in IHC double and triple stainings of monoclonal mouse antibodies
Van der Loos CM, Das PK, Van den Oord JJ, Houthoff HJ, 1989.
Multiple immunoenzyme staining techniques. Use of fluoresceinated, biotinylated and unlabelled monoclonal antibodies.
J Immunol Methods 117, 45-52.
Anti-Biotin and anti-Digoxin in triple immunofluorescence staining
Härtig W, Brückner G, Brauer K, Seeger G, Bigl V, 1996.
Triple immunofluorescence labelling of parvalbumin, calbindin-D28k and calretinin in rat and monkey brain.
J Neurosci Methods. 67(2), 89-95.
Anti-Biotin vs. Streptavidin
Vincent P, Samuel D, 1993.
A comparison of the binding of biotin and biotinylated macromolecular ligands to an anti-biotin monoclonal antibody and to streptavidin.
J Immunol Methods. 165(2), 177-82.
